-
BIRAC Clinical Trial Network is the national peak body that supports and represents networks of clinicians and researchers conducting quality clinical trials and maintaining clinical registries in INDIA
-
Until BIRAC - CTN, there was no single, coordinated mechanism to connect clinical researchers with governments, healthcare policymakers and consumers on issues that impact the conduct of clinical trials across India.
-
Our activities represent the priorities required to develop, implement and support a national framework to expand the capacity, capability, efficiency and effectiveness of clinical trials and clinical quality registries.
-
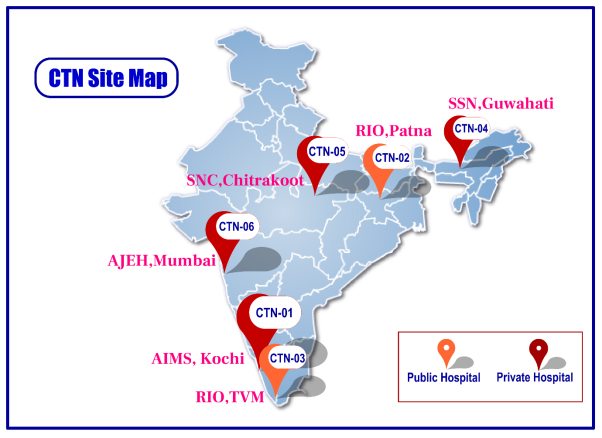
Man power and capacity building for a robust network and data collection for a vast and diverse registry is ongoing at the 6 primary collaborating sites approved by BIRAC across India.
-
Step by step, addition of new sites to the network will be undertaken to establish the biggest registry in India for Ophthalmology.
About Us

Our Mission
As a network of like-minded individuals from the Clinical Research domain who believe in team work, shared knowledge and patient advocacy, we are committed to transform and positively impact our community by helping in the development of new medications and new indications for existing medications for a variety of ophthalmic disorders by creating a Multicentric Registry, capacity building and readiness for clinical trial.
Our Vision
Our vision is to have a flourishing community which is healthy, jubilant and well informed through accurate and high-quality clinical research by our dedicated and experienced multidisciplinary research team.
The need for clinical trial network
| 1 | Growing Biologics Pipeline | India has one of the biggest product pipeline, mostly for diabetes, RA, oncology, Ophthalmology |
| 2 | Lack of GCP Compliant sites | Current trial sites are insufficient for biotherapeutics product development |
| 3 | Lack of Robust registries | Data captured in ICMR(India Council of Medical Research) registries are not always useful for Trials |