-
-
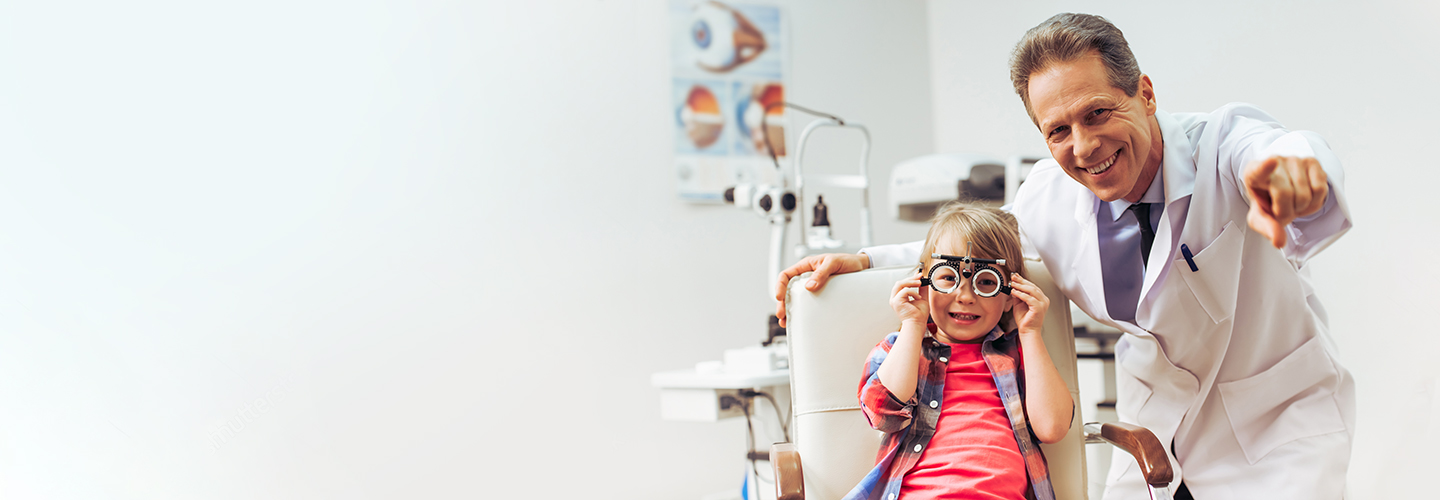
Have you checked
your child's eyes?
Contact us today for an appointment with retina consultant
+022-24177600/13 -

Placing passion
for a noble causeDr. S. Natarajan accepting the Padma Shri for
his humanitarian effort.

